Heart disease is the number one cause of death for both men and women in the United States each year. Our research focuses on using cell-material interactions and localized drug delivery to guide stem cell differentiation for cardiac regeneration and enhance endothelialization of intravascular devices. By improving our ability to repair the damaged or diseased heart, we can decrease the number of patients for which a transplant is necessary and give those not eligible for a transplant the opportunity for a better quality, and possibly longer, life.
Cardiac Regeneration
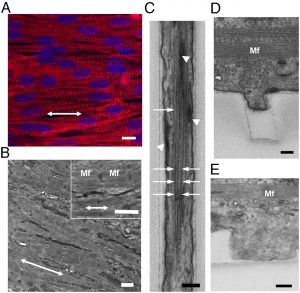
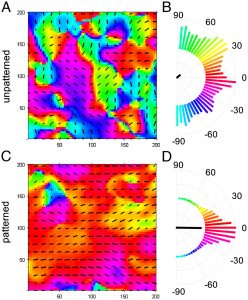
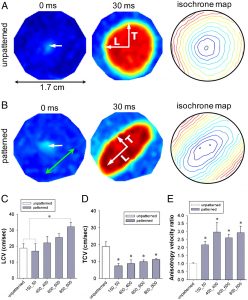
The Lipke Lab is currently developing scalable culture systems that direct differentiation of pluripotent stem cells into cardiomyocytes while maximizing the electrophysiological homogeneity of the resulting cells and minimizing bath-to-batch variability. Characterizing and directing the late-stage electrophysiological characteristics of stem cell-derived cardiomyocytes both at the single cell and the tissue level is important for clinical application of these cells to enhance cardiac regeneration.
Enhancing Endothelialization
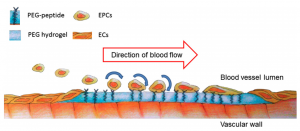
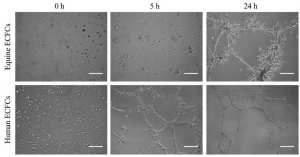
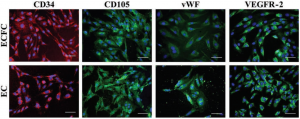
In a healthy blood vessel, a single layer of endothelial cells (ECs) lines the wall of the vessel, separating the blood from the underlying smooth muscle cells in the vessel wall and promoting vascular health by secreting factors such as nitric oxide, which is responsible for vasorelaxation. Promoting endothelialization following the placement of intravascular devices, such as vascular grafts and stents, will improve long-term patency of these devices and reduce restenosis, thereby decreasing the need for repeat procedures and patient morbidity. Endothelial progenitor cells (EPCs), or more specifically human blood outgrowth endothelial colony forming cells, are a promising potential autologous cell source for these applications. The Lipke Lab is investigating the specific cell-material interactions required for ECs and EPCs to populate a biomaterial surface under static and shear conditions and to subsequently create a functional endothelium. We are then applying this knowledge in the design of new co-polymer materials.
Bioengineered 3D Cancer Models
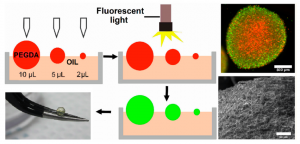
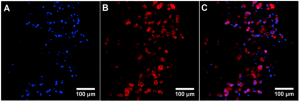
Microenvironmental regulation of tumor progression and metastasis is an important area of focus in current cancer research. Specifically, cancer cells cultured within 3D biomimetic matrices exhibit upregulated tumorigenicity and invasiveness compared to traditional 2D cultured cells and those in multicellular aggregates (tumor spheroids). To this end, the Lipke Lab has developed novel 3D biomimetic models of cancer that can closely replicate key features of the native tumor microenvironment, thereby facilitating the study of 3D tumor progression and metastasis and testing anti-cancer drug efficacy in pre-clinical settings. Using PEG-based hydrogel materials, the Lipke Lab has developed a “tumor millibead” model that can simulate millimeter-scale tumors and a “tumor microsphere” model that replicate micron-sized tumors. In addition, the Lab is also working on microfluidic lab-on-a-chip devices for testing anti-cancer drug efficacy and coculture models of different cancer types (breast, colon, prostate).